The movement of electrons between the shells is called electron transitions.
The emission and absorption spectra are both the result of electron transitions, they can be used like bar codes to identify the different elements.
When electron transitions take place the energy emitted can be detected and its wavelength measured. This provides information about the relative energies of the energy shell.
One packet of energy or photon is released for each electron transition.
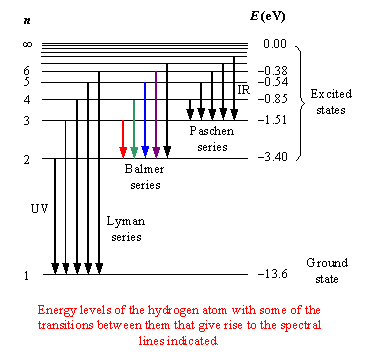
image and the following information from http://eilat.sci.brooklyn.cuny.edu/cis1_5/Old%20HWs/HW2d_C.htm
The emission and absorption spectra are both the result of electron transitions, they can be used like bar codes to identify the different elements.
When electron transitions take place the energy emitted can be detected and its wavelength measured. This provides information about the relative energies of the energy shell.
One packet of energy or photon is released for each electron transition.

image and the following information from http://eilat.sci.brooklyn.cuny.edu/cis1_5/Old%20HWs/HW2d_C.htm
Electrons in their shells can receive energy in the form of heat or electricity and jump to higher energy shells (promotion). They cannot remain at these higher levels (excited state) for very long and soon fall back to their original shell (or other shells). When they fall back (relax) they have to lose the energy difference between the two shells. his loss of energy is performed by releasing electromagnetic energy in the form of infrared, visible light or ultraviolet radiation.
In the hydrogen atom (the simplest case with only one electron to 'jump' between shells) the energy emitted appears in several series of lines each series corresponding to electrons falling back to different levels. This is shown in the diagram below.
The Lyman series corresponds to transitions between the higher shells and the lowest shell (ground state).
The energy shells are usually given a letter 'n' to describe the specific energy level. The lowest level is n=1 the second level is n=2 etc.
The energy shells are usually given a letter 'n' to describe the specific energy level. The lowest level is n=1 the second level is n=2 etc.
Transitions from higher shells (n >2) to n=2 produce radiation in the visible region of the spectrum and we can actually see it by splitting the light using a prism or diffraction grating and projecting it onto a screen.
When an electron falls from a lower to a higher energy level, energy is absorbed and a line in the absorption spectrum is produced.
When an electron falls from a higher to a lower energy level radiation is given out by the atom and a line in the emission spectrum is produced.
When an atom is at the highest energy n=∞, it is no longer in the atom, it has been ionized.
Ionization energy - energy needed to remove an electron from the ground state of each atom in a mole of gaseous atoms.
When an electron falls from a lower to a higher energy level, energy is absorbed and a line in the absorption spectrum is produced.
When an electron falls from a higher to a lower energy level radiation is given out by the atom and a line in the emission spectrum is produced.
When an atom is at the highest energy n=∞, it is no longer in the atom, it has been ionized.
Ionization energy - energy needed to remove an electron from the ground state of each atom in a mole of gaseous atoms.
No comments:
Post a Comment