The masses of the different isotopes and their relative abundance can be measured using a mass spectrometer.
5 Basic Operations - V.I.A.D.D
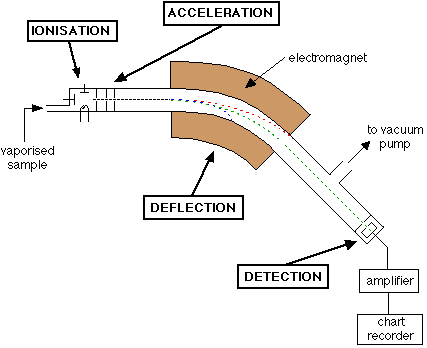
image from http://www.wou.edu/las/physci/taylor/gs331/mass_spec.html
5 Basic Operations - V.I.A.D.D
- Vaporization - Vaporized sample is injected so individual atoms can be analysed.
- Ionization - Atoms are hit with electrons which knock out other electrons producing cations.
- Acceleration - Positive ions are attracted to negatively charged plates. They are accelerated by an electrical field and pass through a hole in the plate.
- Deflection - Accelerated cations are deflected by a magnetic field placed at right angles to their path. Ions that are deflected more are the ones with the smaller mass/charge ratio.
- Detection - cations of a particular mass/charge ratio are detected and a signal is sent to a recorder. The strength of a signal is a measure of the number of ions with that particular ratio that are being detected.

image from http://www.wou.edu/las/physci/taylor/gs331/mass_spec.html
No comments:
Post a Comment